CDSCO License Consultant
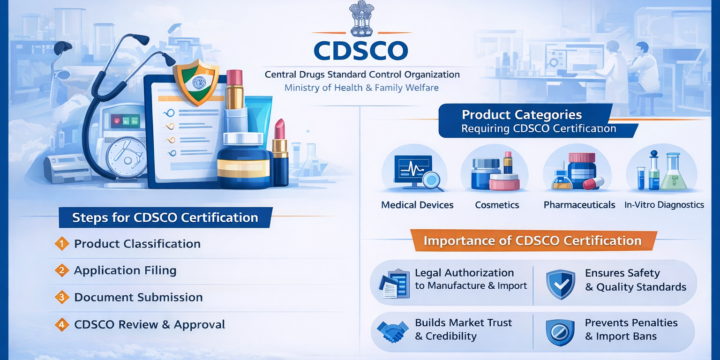
India’s regulatory environment for medical devices, cosmetics, and pharmaceuticals is designed to ensure consumer safety and product quality. The Central Drugs Standard Control Organization (CDSCO) plays a vital role in regulating these sectors. Due to the complexity of procedures, businesses often require a professional CDSCO license Consultant to manage approvals efficiently. With expert regulatory support, companies can avoid delays and ensure full compliance. Platforms like ElectronicsIndia highlight the growing importance of regulatory awareness and compliance consulting for businesses entering India’s regulated markets. You can contact the experts of ElectronicsIndia , who will help you out in providing Cosmetic license. A CDSCO license consultant helps businesses comply with Indian regulatory requirements for drugs, cosmetics, and medical devices. With expert guidance on documentation, registration, and approvals, consultants ensure faster licensing, reduced errors,…